The Advantages of Continuous Assays in Kinome Profiling
Most profiling platforms stop the reaction and read a single time point.
That snapshot assumes the enzyme stayed perfectly linear — but in reality, enzyme activity is dynamic. As a result, endpoint assays can distort potency, overlook time-dependent inhibition, and hide mechanistic behaviors that influence drug discovery outcomes.
The Limitations of Endpoint Kinase Assays
Although endpoint assays remain widely used, they measure only a single timepoint after the reaction has stopped — providing an incomplete picture of enzyme behavior and masking key kinetic information. It’s like judging a movie from a single frame, missing critical information.
Endpoint assays capture data only after the enzyme reaction is halted, offering a static snapshot that fails to reflect how activity evolved over time.
They rely on the assumption that enzyme activity stays constant throughout the reaction, even though most enzymes exhibit changing rates as substrates deplete or inhibitors engage.
Solubility issues, compound aggregation, or assay interference can distort endpoint signals, creating false impressions of inhibition or activation.
Critical kinetic behaviors — such as lag phases, slow-binding inhibition, or reversible interactions — remain invisible, preventing a full understanding of inhibitor mechanism.
Continuous assays overcome these challenges by capturing enzyme activity in real time rather than at a single endpoint.
Instead of inferring behavior from a static value, they directly monitor reaction progress — revealing how activity changes second by second as substrates are converted and inhibitors interact.
This approach provides a full kinetic profile that exposes subtle but critical behaviors — such as time-dependent inhibition, reversibility, and off-rate effects — delivering data that truly reflect enzyme mechanism.
Continuous Assays Capture True Enzyme Behavior
A Single Timepoint Can’t Tell the Whole Story
AssayQuant’s PhosphoSens® technology continuously measures enzyme activity in real time — directly tracking substrate phosphorylation rather than relying on indirect coupling reactions.
Each well produces a complete progress curve with dozens of timepoints, revealing exactly how enzyme activity changes throughout the reaction. This allows for accurate, rate-based % inhibition values tailored to each of the 400+ wild-type kinases profiled in our KinSight™ selectivity screens.
Unlike endpoint assays that provide a single snapshot, the PhosphoSens continuous format captures the entire story — every frame of enzyme behavior. By measuring catalytic activity kinetically, it reveals true selectivity and mechanism of action, giving researchers the mechanistic clarity needed to drive drug development forward with confidence.
This approach forms the foundation of AssayQuant’s KinSight Profiling Service, which delivers kinetic insight into kinome-wide selectivity and mechanism of action (MOA) — helping discovery teams make data-driven decisions about therapeutic opportunities and off-target liabilities with greater confidence and precision.
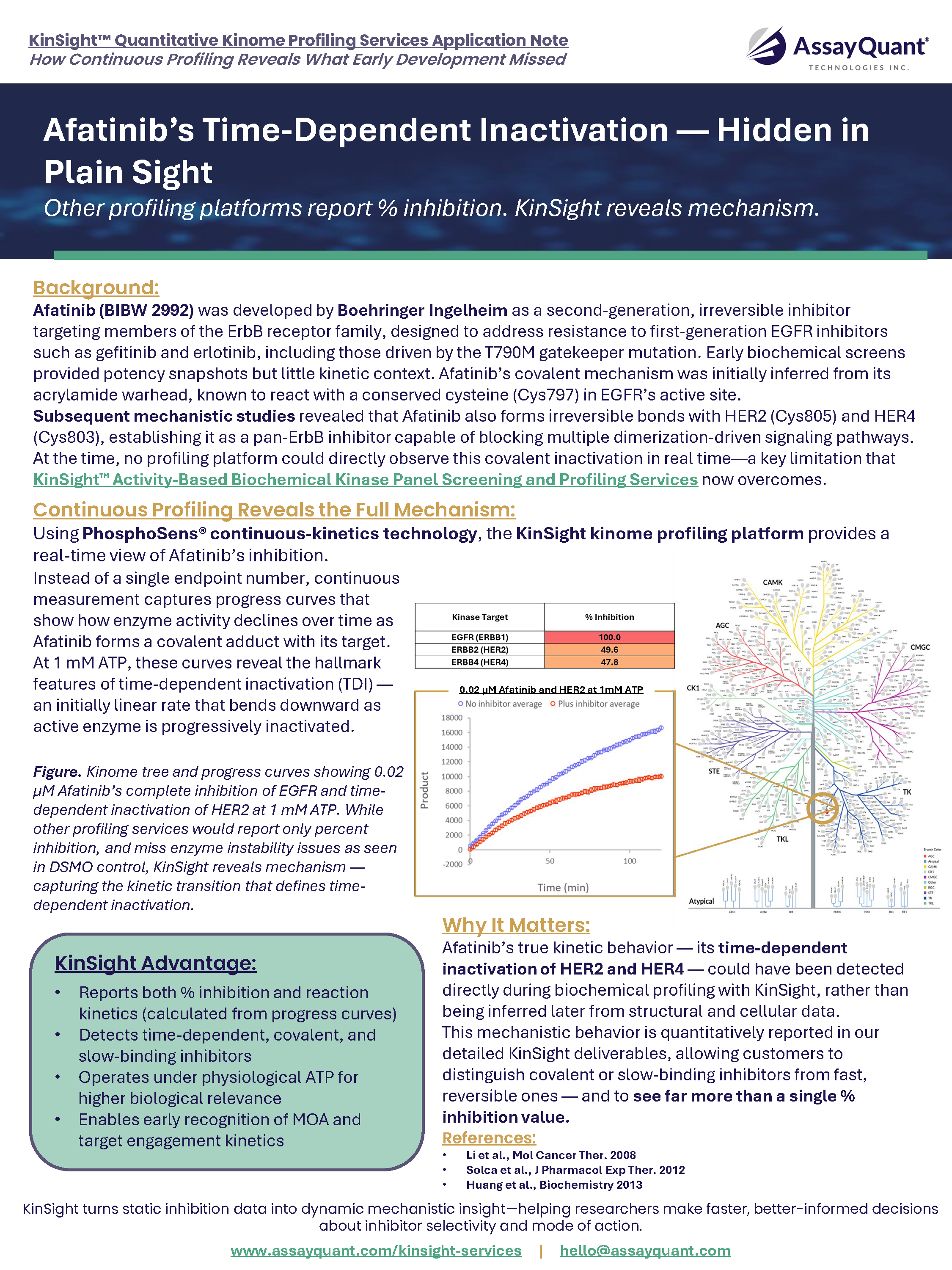
Afatinib’s Time-Dependent Inactivation — Hidden in Plain Sight
Learn how KinSight reveals Afatinib’s covalent pan-ErbB mechanism that other profiling platforms missed.
What Continuous Profiling Reveals That Endpoints Miss
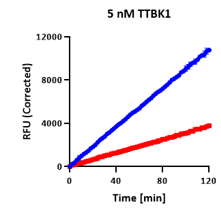
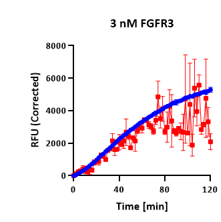
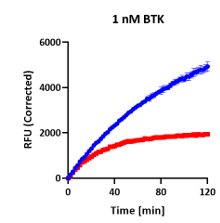
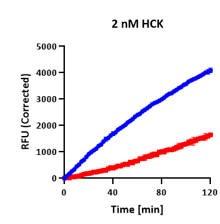
AssayQuant’s Quartet: Same Endpoint, Different Story
In a comparative example, four kinases — TTBK1, FGFR3, BTK, and HCK — each appeared to show ~60–65% inhibition in a standard endpoint assay.
Each mechanism produces similar endpoint values — yet radically different kinetics and implications for inhibitor optimization.
On paper, they look the same, but continuous progress curves tell a very different story:
| Target | % Inhibition |
| TTBK1 | 65% |
| FGFR3 | 61% |
| BTK | 61% |
| HCK | 60% |
"AssayQuant's PhosphoSens technology is uniquely suited to advance Vibliome's kinase inhibitor program. PhosphoSens provides a deep understanding of our compounds including specificity, potency, mode-of-inhibition, reversibility, and time-dependent inhibition. This, along with AssayQuant's world-class scientific support and fast turnaround times powered by automation, will make this a highly productive partnership that will accelerate Vibliome's drug development programs."
PhosphoSens: Direct Measurement of True Catalytic Activity
Continuous assays track catalytic activity in real time, eliminating the need to assume reaction linearity. It yields an actual reaction rate determined from dozens of data points, generating a full progress curve in every well, enabling accurate rate-based % inhibition values grounded in actual enzymatic behavior.
By capturing the full reaction progress curve, continuous assays reveal subtle kinetic behaviors such as time-dependent inhibition, partial reversibility, or slow-binding effects. These insights help distinguish between compounds with similar endpoint inhibition but very different mechanisms of action.
PhosphoSens assays can be performed at physiological ATP concentrations & cofactor conditions and use peptide substrates derived from biologically relevant sequences to measure enzyme activity directly. The resulting data are both reproducible and physiologically meaningful.
Continuous assays provide mechanistic clarity across hundreds of kinases, allowing researchers to identify off-target liabilities early and prioritize the most promising therapeutic leads. This data-driven understanding accelerates optimization and reduces the risk of late-stage failure.
Ready to see how continuous kinome profiling can guide your inhibitor development?
Whether you’re optimizing a lead compound or exploring kinase selectivity across hundreds of targets, KinSight™ continuous profiling delivers the mechanistic clarity you need to make confident, data-driven decisions.
Stay Informed
Want to hear the latest about our technology? Be among the first to learn about our latest products and services.