PhosphoSens-Red Kinase Assays
Reaction conditions and experimental protocols for PhosphoSens-Red Kinase Assays.
Reaction Conditions and Setup
Kinase assays that provide a direct measure of catalytic function are paramount for pre-clinical drug discovery success. Although limitations exist in establishing an enzyme assay for a new kinases, AssayQuant is striving to reduce this limitation to enable the quantum improvement needed to accelerate creation of more effective therapeutics.
With this goal in mind, access information below to ensure you are performing your assay with under optimized conditions.
For target specific assay validation data, please inquire at support@assayquant.com.
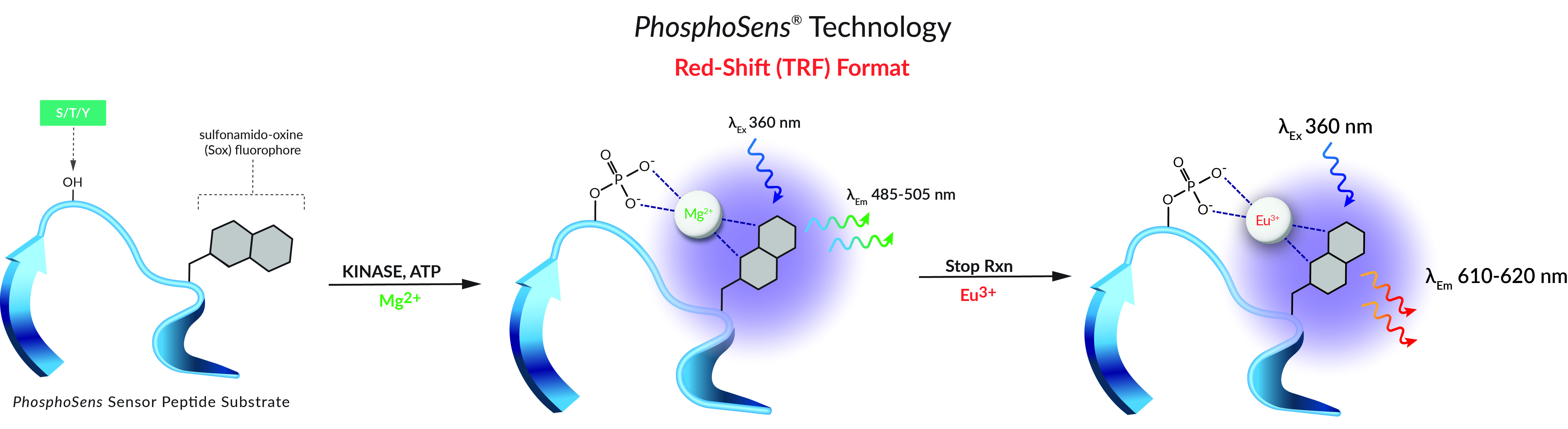
General Kinase Reaction Conditions
Many kinase assays can be performed under general kinase reaction conditions. Our general kinase reactions include the following components.
- 54 mM HEPES, pH 7.5
- 1 mM ATP or ATP Km
- 1.2 mM DTT
- 0.012% Brij-35
- 1% glycerol
- 0.22 mg/ml BSA
- 0.55 mM EGTA
- 10 mM MgCl2
- 10-15 μM Sensor Peptide
- 0.05-5 nM Kinase (adjusted as needed)
- Additional co-factors or additives (as required)*
*Please reference the target-specific conditions table below to determine whether your target requires any additives or co-factors for optimal performance.
Target-Specific Reaction Conditions
Some kinases require additives or co-factors for optimal performance. Please reference the target-specific conditions table below to see what, if any, additional reagents are recommended.
| Target(s) HGNC (Common) Name |
Component | Description | Final Concentration of Additive in Assay |
| CAMK1 (CAMK1α) | Calcium Calmodulin Solution, 10X |
50 ng/µl Calmodulin, 4 mM CaCl2, 1 M Tris-HCL, pH 7.0, 1 M Tris-HCl, pH 7.5 in nuclease free water
|
5 ng/µl Calmodulin
0.4 mM CaCl2
5mM TRIS-HCl, pH7
5mM TRIS-HCl, pH 7.5
(-) 0.55 mM EGTA
|
|
PNCK (CAMK1β)
|
|||
|
CAMK1D (CAMK1δ)
|
|||
|
CAMK1G (CAMK1γ)
|
|||
|
CAMK2A (CAMK2α)
|
|||
|
CAMK2B (CAMK2β)
|
|||
|
CAMK2D (CAMK2δ)
|
|||
|
CAMK2G (CAMK2γ)
|
|||
|
CAMK4
|
|||
|
CAMKK1
|
|||
|
DAPK1
|
|||
|
DAPK2 (DRP‐1)
|
|||
|
DAPK3 (ZIPK)
|
|||
|
DCLK1 (DCAMKL1)
|
|||
|
DCLK2 (DCAMKL2)
|
|||
|
EEF2K (eEF2K)
|
|||
|
MYLK (MLCK)
|
|||
|
MYLK2
|
|||
|
PHKG1
|
|||
|
PHKG2
|
|||
|
EIF2AK4 (GCN2)
|
10µg/mL t-RNA
|
10µg/mL t-RNA
|
1µg/mL t-RNA
|
|
PDK1 (PDHK1)
|
KCI Solution, 100 mM
|
100mM KCl in nuclease free water
|
10 mM KCl
|
|
PDK2 (PDHK2)
|
|||
|
PDK3 (PDHK3)
|
|||
|
PDK4 (PDHK4)
|
|||
|
PRKAA1‐B1‐G1 (AMPKα1β1γ1)
|
10 mM AMP
|
10 mM AMP
|
1 mM
|
|
PRKAA1-B1-G2 (AMPKα1β1γ2)
|
|||
|
PRKAA1-B1-G3 (AMPKα1β1γ3)
|
|||
|
PRKAA1‐B2‐G1 (AMPKα1β2γ1)
|
|||
|
PRKAA1-B2-G2 (AMPKα1β2γ2)
|
|||
|
PRKAA1-B2-G3 (AMPKα1β2γ3)
|
|||
|
PRKAA2‐B1‐G1 (AMPKα2β1γ1)
|
|||
|
PRKAA2-B1-G2 (AMPKα2β1γ2)
|
|||
|
PRKAA2-B1-G3 (AMPKα2β1γ3)
|
|||
|
PRKAA2‐B2‐G1 (AMPKα2β2γ1)
|
|||
|
PRKAA2-B2-G2 (AMPKα2β2γ2)
|
|||
|
PRKAA2-B2-G3 (AMPKα2β2γ3)
|
|||
|
PRKCA (PKCα)
|
PS/DAG Solution, 10X
|
1.4 mM L-α-phosphatidylserine , 0.038 mM 1-2-dioleoyl-sn-glycerol, 1 M HEPES, pH 7.5 in nuclease free water
|
140 µM Phosphatidylserine
3.8 µM Diacylglycerol
(-) 0.55 mM EGTA
|
|
PRKCB1 (PKCβ1)
|
|||
|
PRKCB2 (PKCβ2)
|
|||
|
PRKCD (PKCδ)
|
|||
|
PRKCE (PKCe)
|
|||
|
PRKCG (PKCγ)
|
|||
|
PRKCH (PKCη)
|
|||
|
PRKCI (PKCι)
|
|||
|
PRKCQ (PKCᶿ)
|
|||
|
PRKCZ (PKCζ)
|
|||
|
PRKDC (DNA‐PK)
|
500µg/mL DNA
|
500µg/mL DNA
|
1µg/mL t-RNA
|
|
PRKG1 (PKG1, PGK)
|
cGMP Solution, 10 mM
|
10 mM cGMP in nuclease free water
|
1 mM cGMP
|
|
PRKG2 (PKG2, PGK2, CGK2)
|
|||
|
RIPK1
|
MgCl₂ Solution, 100mM
|
100 mM MgCl₂ in nuclease free water
|
10 mM MgCl₂
|
|
RIPK2
|
|||
|
RIPK3
|
|||
|
RIPK5
|
|||
|
BCKDK
|
KCI Solution, 500 mM
|
500mM KCl in nuclease free water
|
50 mM KCl
|
|
MAP3K6
|
N/A
|
N/A
|
(-) 1.2 mM DTT
|
Preparing Reagents
Prior to setting up the individual reactions, prepare the following solutions:
- PhosphoSens Sensor Peptide Substrate (1 mM):
Bulk PhosphoSens sensor peptides are supplied as 1 mg lyophilized powder. Dissolve the sensor peptide substrate as indicated on the vial or in the Technical Notes section of the Certificate of Analysis to create the 1 mM stock. If the sensor peptide requires the addition of dimethyl sulphoxide (DMSO), add the DMSO first to ensure solubility followed by the aqueous component, gently vortexing the solution between additions to ensure complete dissolution.
Sensor peptides that are provided in the PhosphoSens Evaluation Kits, are already solubilized as a 1 mM solution in the solvents defined in the Technical Notes section of the Certificate of Analysis.
- PhosphoSens Sensor Peptide Substrate Solution (100 µM):
Sensor peptides that are provided in the PhosphoSens Kits, are already solubilized as a 1 mM solution in the solvents defined in the Technical Notes section of the Certificate of Analysis.
Prepare 0.1 mM Substrate solution by thawing the 1 mM peptide substrate stock solution, mixing well by vortexing gently, removing an appropriate amount and diluting 10-fold into ultrapure deionized water.
- Adenosine 5′-triphosphate disodium salt hydrate (ATP) Solution (10 mM):
ATP is supplied as a 100mM stock solution. Prepare 10 mM ATP solution by adding 50 μL of 100 mM ATP to 450 μL ultrapure deionized water.
NOTE: These instructions are for running the assay at 1 mM ATP. If running the assay at ATP Km, please prepare 500 µL of a stock of ATP at 10X the ATP Km.
- DL-Dithiothreitol (DTT) Solutions (10 mM):
DTT is supplied as 1M stock solution. Prepare 10 mM DTT solution by adding 5 μL of the 1 M DTT to 495 μL ultrapure deionized water.
CAUTION: Diluted DTT is readily oxidized so use fresh dilutions.
- Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) Solution (5.5 mM):
EGTA is supplied as 550mM stock solution. Prepare 5.5 mM EGTA solution by adding 5 μL of 550mM EGTA to 495 μL ultrapure deionized water.
- Enzyme Reaction Buffer (ERB, 10X):
ERB is provided as a 10X stock solution. ERB is added directly to the ‘Master Mix’.
- Enzyme Dilution Buffer (EDB, 5X):
EDB is provided as a 5X stock solution. EDB is added to Blank or Background wells as a "No Enzyme" control and is used to dilute enzymes.
- Europium (30 mM):
Europium is provided as a 30 mM stock solution. This can be added directly to each well to a final concentration of 5 mM.
Preparing ‘Master Mix’
After preparing reagents as described above, prepare an appropriate amount of 'Master Mix' for your experiments.
The individual components and respective volumes to prepare 0.875mL of ‘Master Mix’ for a general kinase reaction at 1 mM ATP is outlined below:
|
COMPONENT |
VOLUME |
|
Enzyme Reaction Buffer (10X) |
125 μL |
|
ATP Solution (10 mM) |
125 μL |
|
DTT Solution (10 mM) |
125 μL |
|
EGTA Solution (5.5 mM) |
125 μL |
|
Water |
375 μL |
PhosphoSens-Red Kinase Reaction Set-Up
Reaction Set-Up outlined below is written for a 20 μL total volume/well in 384-well plate and 10 μM sensor peptide:
Step 1. Add 2 μL 100 μM Sensor Peptide
Step 2. Add 14 μL ‘Master Mix’ containing reaction buffer, ATP, DTT, and EGTA (and additional co-factors or additives, if needed)
Step 3. Pause for 5-minute incubation at 30°C
Step 4. Add 4 μL 5X Enzyme dilution buffer or 5X Kinase in EDB
Step 5. Add plate to reader and monitor kinase activity by collecting fluorescence intensity (RFU) readings (lExMax 360 nm/lEmMax ~492 nm [485-498 nm]) every 0.5-2.0 minutes at 30°C until the progress curve of the no-inhibitor control reaches the top the linear range.
Step 6. Remove plate from reader and remove plate seal.
Step 7. If your target is a serine/threonine kinases, process to step 8.
If your target is a tyrosine kinase, add 5 µL of 0.5 M NaOH to each well and incubate for 5 minutes at room temperature to adjust the pH.
Step 8. Add 4 µL of the 30 mM Europium solution to each well, for a final Europium concentration of 5 mM, and incubate for 5 minutes at room temperature.
Step 9. Return the plate to the reader and take an endpoint time-resolved fluorescence (RFU) reading (lExMax 360 nm/lEmMax ~620 nm) with the plate unsealed.
Experiment Specific Protocols
Stay Informed
Want to hear the latest about our technology? Be among the first to learn about our latest products and services.