The Core Technology Behind AssayQuant’s Real-Time Enzyme Assays
PhosphoSens® is AssayQuant’s patented fluorescence detection platform for quantifying enzyme activity in real time at the substrate level.
At its core are sensor peptide substrates that fluoresce upon phosphorylation or dephosphorylation — enabling direct, assumption-free measurement of catalytic turnover without coupling enzymes, antibodies, or wash steps.
By linking catalytic function directly to a change in fluorescence, PhosphoSens® reveals how enzymes behave — not just how much they’re inhibited.
Our continuously expanding library of >50,000 optimized Sox-based peptide substrates covers nearly every major kinase and phosphatase family, supporting assays in both recombinant and lysate samples.
The Science Behind PhosphoSens® — The Sox Fluorophore
At the core of every PhosphoSens assay is the Sox (sulfonamido-oxine) fluorophore — a patented innovation developed by the Imperiali Lab at MIT and exclusively licensed by AssayQuant Technologies.
Unlike conventional fluorophores, Sox is an active sensor: it reports phosphorylation directly through a change in fluorescence intensity, providing a real-time, quantitative readout of enzymatic activity.
This unique chemistry is what enables the PhosphoSens platform to deliver continuous, direct measurements of kinase and phosphatase activity — without coupling enzymes, antibodies, or wash steps.
Signal mechanism |
Chelation-enhanced fluorescence (direct measure of activity) |
|---|---|
Size / Hydrophobicity |
Small, non-hydrophobic |
Data Type |
Continuous (real-time) or endpoint (TRF) |
Artifact Risk |
Minimal — designed to avoid compound interactions |
Example Outcome |
Accurate kinetic data (Ki, kinact/KI, residence time) |
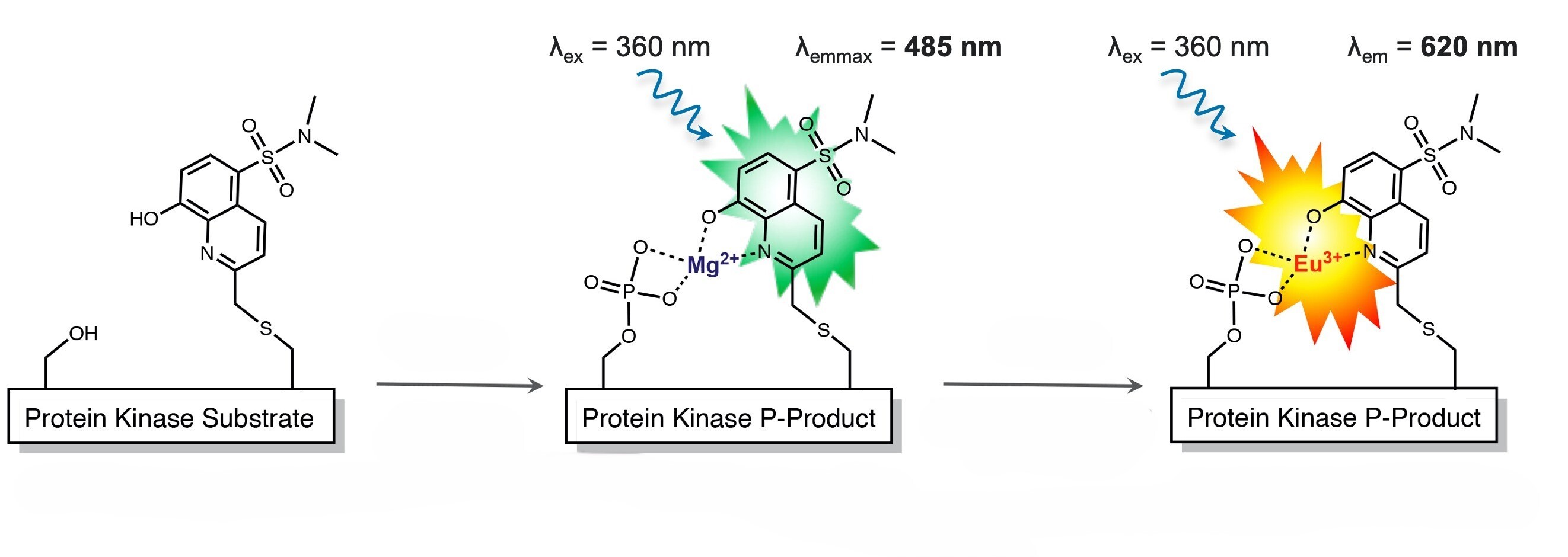
Engineering Physiological Peptide Sensors for True Enzyme Function
While the Sox fluorophore is the signaling engine of the PhosphoSens platform, the peptide sequence surrounding it allows us to fine tune target specificity and selectivity.
AssayQuant is the only company that systematically designs and validates physiologically relevant peptide substrates for kinase and phosphatase activity — ensuring that every sensor faithfully reflects the true enzyme–substrate interaction.
High-Throughput Peptide Sensor Design
Each PhosphoSens sensor peptide is synthesized using a proprietary Fmoc-Cys-Sox (CSx) building block, allowing precise placement of the Sox fluorophore near the phosphorylation site.
Using high-throughput solid-phase synthesis, we screen thousands of peptide variants in parallel to identify those that most closely mimic natural substrate motifs.
Our library now exceeds 50,000 validated CSx-based sequences, covering virtually every major kinase and phosphatase family.
Optimized peptides exhibit enhanced catalytic turnover, higher sensitivity, and improved selectivity — enabling reproducible, quantitative assays for even difficult targets.
Native Peptide Sequences. Real Enzyme Behavior.
Most commercial kinase assays rely on heavily modified or artificial substrates — often containing added lysines, arginines, or bulky reporter groups that distort native enzyme behavior.
These modifications can interfere with substrate recognition, mask true inhibitor potency, and introduce hydrophobic artifacts that confound screening outcomes.
PhosphoSens substrates are derived from authentic biological peptide sequences, preserving the native phosphorylation context found in living systems.
The result: data that accurately reflect in-cell activity, enabling more confident decisions during discovery, profiling, and optimization.
Two Complementary Formats — Built on the PhosphoSens Platform
PhosphoSens technology powers two complementary assay formats that give researchers the flexibility to study enzyme activity in the way that best fits their workflow.
Both formats rely on the same core chemistry — fluorescent sensor peptide substrates that directly couple catalytic turnover to a measurable fluorescence signal — but differ in how the signal is captured and analyzed.

Directly Measures Activity at the Substrate Level

Simple "Add-and-Read" Workflow

Validated in 96-, 384-, and 1536-Well Plates

Compatible With Standard Plate Readers

No Coupling Enzymes or Antibodies Required
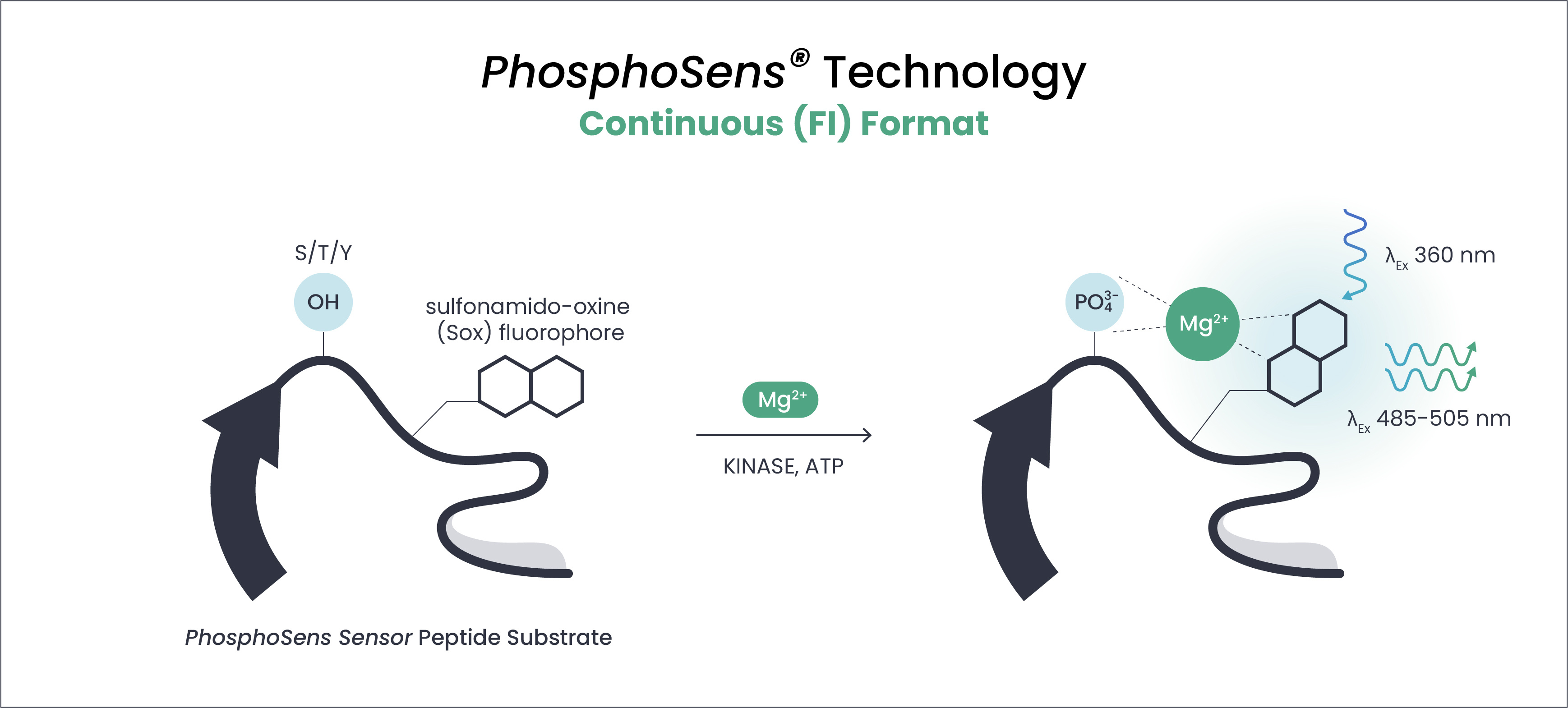
Continuous, Kinetic Fluorescent Intensity (FI) Assays
PhosphoSens-Kinetic
Applications: Basic research through lead optimization
Description: Continuously measure catalytic activity in real time — revealing how inhibition occurs, not just how much. Each well produces a progress curve from which true initial velocities (V₀), time-dependent inhibition, and other mechanistic parameters can be extracted.
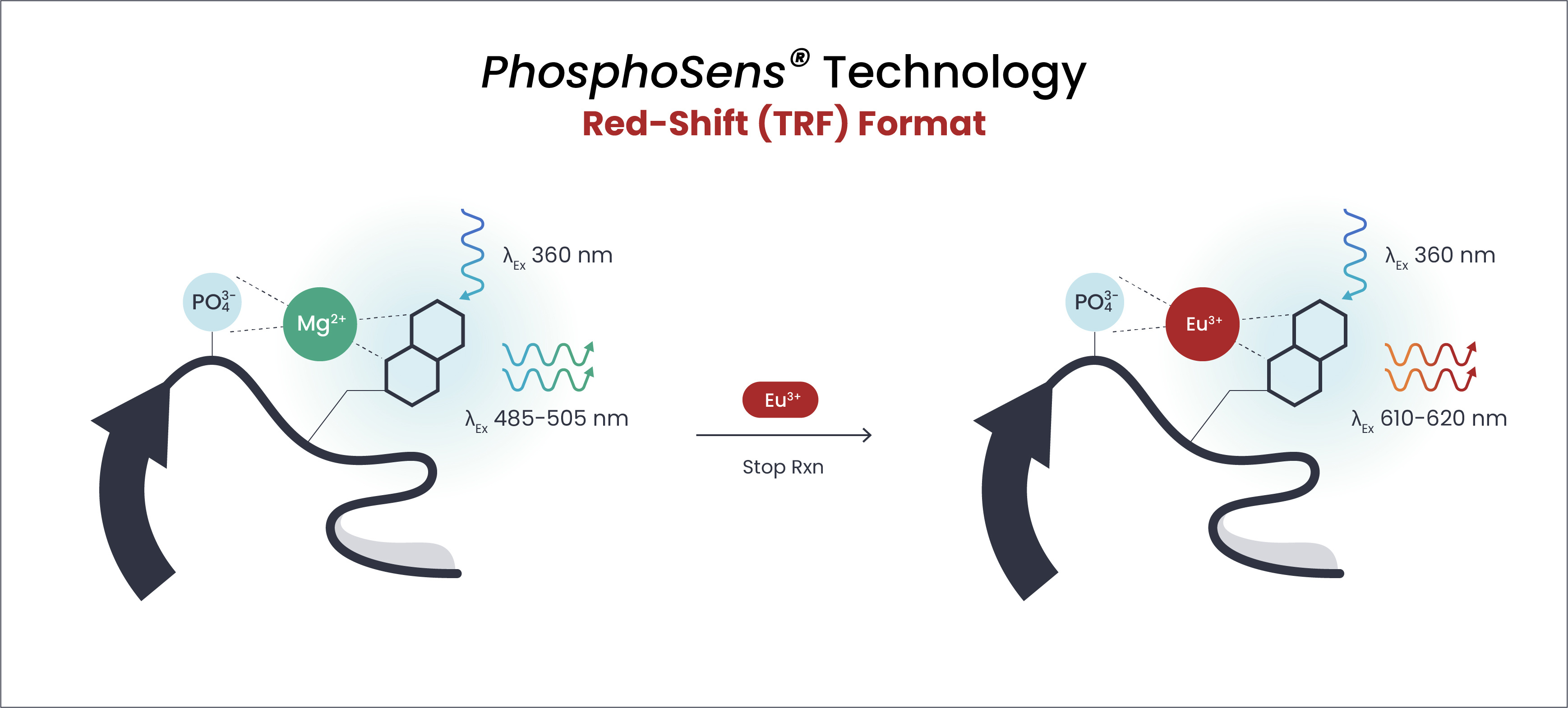
Endpoint, Time-Resolved Fluorescence (TRF) Assays
PhosphoSens-Red
Applications: High-throughput screening (HTS)
Description: Optimized for primary screening campaigns, PhosphoSens-Red assays combine our substrate-based detection chemistry with a streamlined, mix-and-read TRF format. Ideal for large compound libraries, automated workflows, and rapid ranking of inhibitor potency.
Applicable Enzyme Classes & Sample Types — One Platform, Multiple Possibilities
PhosphoSens technology provides broad versatility — enabling measurement of kinase and phosphatase activity across a wide range of experimental contexts.
Whether working with purified recombinant enzymes or complex lysate samples, researchers can quantify true catalytic function under physiological ATP and cofactor conditions, revealing mechanistic behavior in biologically relevant settings.
Enzyme Classes:
Sample Types:
Protein Kinases
Measure phosphorylation directly at the substrate level to assess enzyme activity, compound selectivity, and inhibitor potency.
PhosphoSens® kinase assays provide true kinetic or single-read data that accurately reflect catalytic turnover and mechanistic behavior under physiologic ATP and cofactor conditions.
Purified Enzymes
Gain deep mechanistic insights using recombinant kinase or phosphatase enzymes under defined buffer, ATP, and cofactor conditions.
Ideal for enzyme kinetics, compound potency determination, and mechanistic characterization where reaction control is critical.
Protein Phosphatases
Quantify dephosphorylation activity in real time to evaluate reversibility, regulatory control, and inhibitor effects.
PhosphoSens® phosphatase assays operate under physiologically relevant Mg²⁺ or Mn²⁺ concentrations, enabling precise measurement of enzymatic activity and pathway modulation.
Cell Lysates & Tissue Homogenates
Directly measure endogenous kinase or phosphatase activity in biologically relevant and complex matrices.
Capture pathway-level activity, inhibitor effects, and regulatory responses in native cellular environments to link biochemical behavior with biological relevance.
From Screening to Kinetics — One Platform, Unified Chemistry
Use PhosphoSens-Red Screening Assays to identify active compounds or probe selectivity, then transition seamlessly to PhosphoSens-Kinetic Activity Assays for quantitative mechanistic insight.
Both leverage the same trusted chemistry — providing continuity from discovery to detailed characterization.
Identify and Validate the Right Candidates Faster with Richer Data
Applications:
- Kinome Profiling: Gain detailed kinetic insights into compound selectivity and mechanisms of action (MOA) by profiling against a comprehensive panel of over 400 wild-type kinases. Experiments can be conducted under physiological conditions with high or low ATP levels to match your research needs.
- IC₅₀ or Kᵢ Determination: Evaluate compound potency using 12-point dose-response curves. Flexible assay conditions include the presence of BSA or DTT, high or low ATP levels, and the ability to analyze protein complexes or crude lysates.
- kinact/Kᵢ for Time-Dependent Inhibition: Quantify covalent compound inhibition dynamics with 24-point time-dependent assays. These provide precise measurements of inactivation rates (kinact) and covalent binding affinities (Kᵢ).
- Residence Time: Characterize compound binding kinetics using the Jump Dilution method, with a detection range from 30 seconds to 48 hours. This service provides valuable data on compound dissociation rates for informed drug discovery.
- Mechanism of Action (MOA): Uncover how your compound interacts with kinases through detailed studies using ATP, peptide, or lipid substrates. Tailored to your target and research goals, MOA assays provide actionable insights.
- EC50: Assess compound potency for target activation with EC₅₀ measurements, delivering reliable data on dose-dependent activation to guide lead optimization.
Indications:
- Cancer: Exploring kinase-driven pathways, such as those involved in uncontrolled cell proliferation and survival, and metastasis.
- Neurological Disorders: Investigating signaling pathways regulated by kinases or phosphatases including Parkinson’s Disease, stroke, and various tauopathies such as Alzheimer's disease (AD), frontotemporal dementia, corticobasal degeneration (CBD), and chronic traumatic encephalopathy, characterized pathologically by the deposition in the brain of aggregates of the insoluble hyperphosphorylated form of the tau protein (p-tau).
- Cardiovascular Diseases: Studying kinases or phosphatases that affect heart function, blood vessel regulation, cardiac hypertrophy, myocardial infarction and hypertension.
- Immunology: Evaluating kinases or phosphatases involved in immune cell activation, chronic inflammation, and autoimmune diseases where signaling is deregulated.
- Metabolic Disorders: Characterize kinases or phosphatases implicated in diabetes, insulin resistance, obesity, dyslipidemia, and metabolic dysfunction-associated fatty liver disease (MAFLD).
The Best Solutions Start with a Conversation
Not sure which product or service is the best fit for your project? At AssayQuant, our team of scientific experts can provide the guidance and support you need to achieve your research goals.
Stay Informed
Want to hear the latest about our technology? Be among the first to learn about our latest products and services.