PhosphoSens® Lysate Kinase Activity Assays
PhosphoSens Lysate Assays directly quantify functional kinase activity in cell and tissue lysates. Unlike phosphoprotein methods that only measure phosphorylation state as a proxy, PhosphoSens captures true enzymatic activity at the substrate level, delivering real-time progress curves in a biologically relevant context.
Every well produces a complete activity profile — not just a snapshot — so you can clearly see kinase function, inhibitor dynamics, and pathway modulation in the context of native biology.
Direct, Real-Time Detection of Kinase Activity in Complex Samples
AssayQuant's Cell Lysate Kinase Assays combine PhosphoSens Detection Technology with high-throughput peptide synthesis to design sensor peptide substrates that are highly selective for your targets of interest.
These Kinase-Selective Lysate Substrates are developed with our readout molecule, Sulfonamido-oxine (Sox), covalently attached via a cysteine residue (native or introduced) in close proximity to the Serine, Threonine, or Tyrosine phosphorylation site (± 2-5 residues).
The Sox fluorophore reports peptide phosphorylation by the target kinase via chelation-enhanced fluorescence (ChEF). The fluorescence-sensing mechanism is based on the chelate effect introduced by phosphorylation of the Ser/Thr/Tyr residue proximal to CSox, which enhances magnesium (Mg²⁺) binding affinity compared to the unphosphorylated species, and results in increased fluorescence emission intensity (2-10-fold).
In this way, CSox-modified peptide substrates provide a fluorescent intensity signal that is directly proportional to the phosphorylation level of the substrate, enabling continuous/real-time kinetic measurements of kinase activity in medium to high throughput (96−1536 well plate).
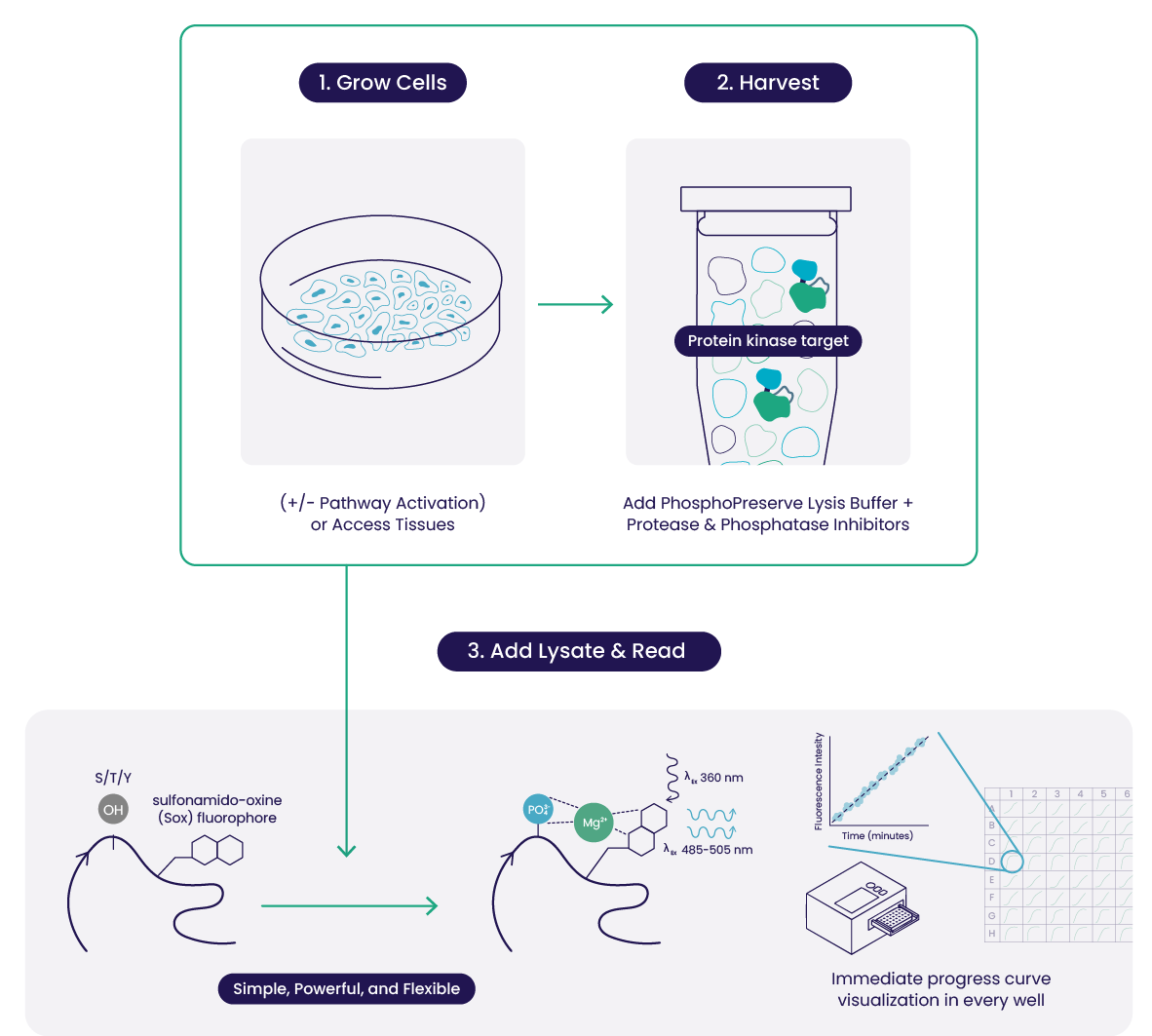
Prepare your Sample
Grow cells (+/- stimulus), aspirate medium and wash, and add PhosphoPreserve Cell Extraction Buffer with Protease and Phosphatase Inhibitors Cocktails to lyse the cells.
Add
Sample is added to reaction mix containing a Sensor Peptide Substrate, catalyzing phosphotransfer from ATP to the substrate in real time.
Read
Add plate to reader and monitor kinase activity throughout the reaction. Signal is directly proportional to the phosphorylation level of the substrate.
Understand How Our Technology Can Support Your Next Discovery
Kinase Activity Across Real Cell Models
See how kinase activity changes across multiple cell lines, conditions, and stimuli — and why functional assays reveal dynamics that phospho readouts miss.
Phosphorylation ≠ Activity
Most kinase assays infer activity from phospho-status. Learn why catalytic function and phosphorylation often diverge — and how PhosphoSens measures real activity.
From Selective Substrates to Functional Insights
Our peptide substrates are engineered to be highly selective, ensuring that kinase activity can be measured directly even in the complexity of unfractionated lysates. This design eliminates interference from other kinases and background phosphorylation, allowing the signal to reflect true catalytic activity. By pairing substrate precision with continuous detection, PhosphoSens® assays turn raw phosphorylation events into clear, functional insights into enzyme behavior and inhibitor response.
Selective Kinase Substrate Development
While our current portfolio of biochemical assays are designed for purified kinases and optimized for activity and sensitivity, running assays in complex samples requires an added layer of selectivity. That’s because lysates contain many active kinases, requiring our new sensors to be engineered to detect the intended target without interference—ensuring accurate, meaningful data in complex sample types. To develop these substrates, we offer Custom Assay Development Services which focuses on a Milestone-based approach.
This careful substrate design ensures that PhosphoSens® assays report true catalytic activity, even in the complexity of cell lysates — a key advantage over phosphoprotein approaches.
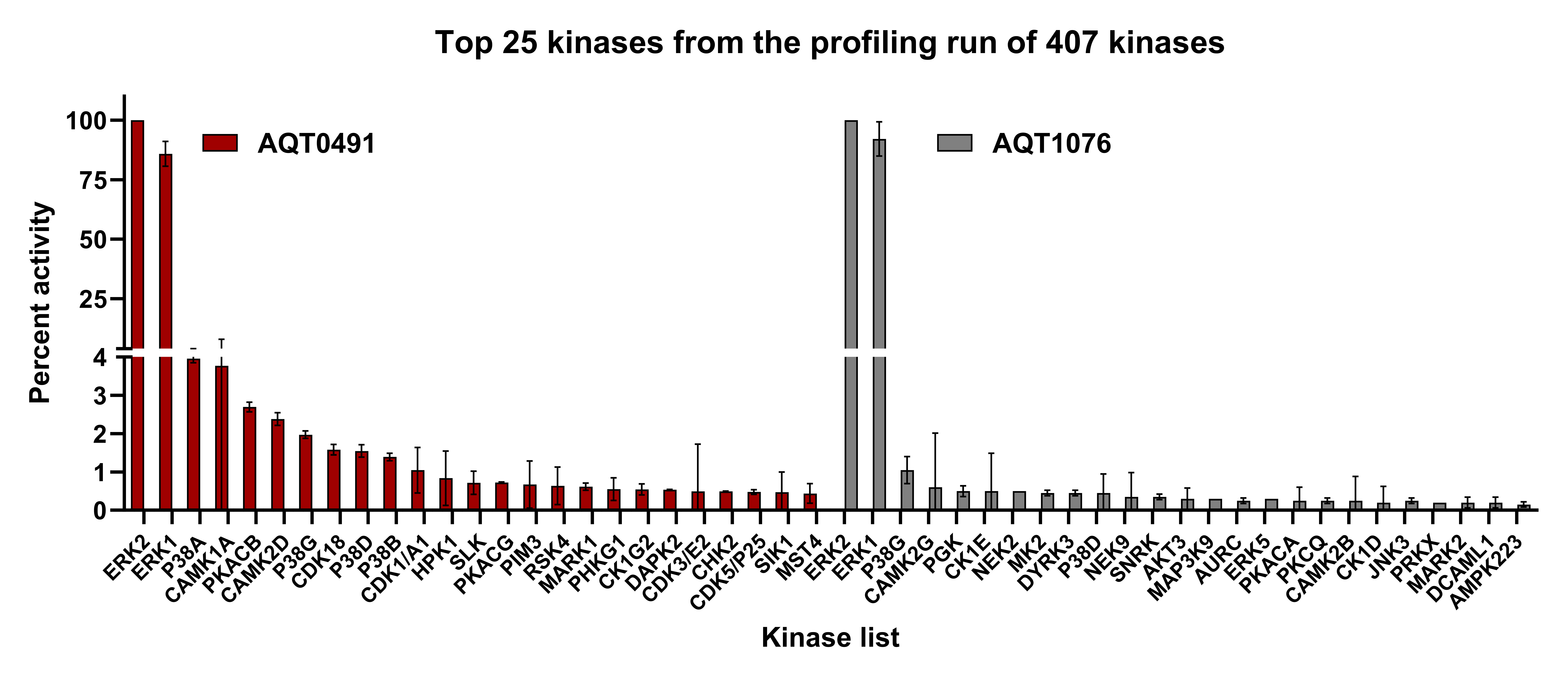
Screened 123 candidate peptides from derived from native substrate sequences.
Identified AQT0491, with high activity but moderate selectivity.
Screened 259 sequences for ERK1/2 activity and selectivity.
Selected optimized single amino acid substitutions that enhance performance.
Combined substitutions to generate 194 peptide variants.
Top-performing peptide, AQT1076, improved selectivity 10x and ERK2 activity 1.5x over AQT0491.
Study Kinase Enzyme Activity Where Biology Happens
Most kinase assays overlook biological complexity.
To address this challenge in protein kinase inhibitor (PKI) development, we designed a kinase activity assay that delivers mechanistic insight and biological relevance.
Download our poster to learn how we are developing Selective Sensor Peptides that directly quantify kinase activity in crude cell lysates and tissue homogenates.
Revolutionizing Kinase Research and Drug Development with Continuous Activity Detection in Complex Biological Samples
By using unfractionated cell lysates or tissue homogenates, our assays let you measure kinase activity in a native, biologically relevant environment. This enables you to examine kinase complexes from multiple sample types and link target inhibition to broader signaling network status and disease relevance.
Unlike simplified biochemical assays, our format maintains the complexity of the cellular milieu, capturing key factors that affect kinase behavior and inhibitor performance in vivo:
Cellular Components
Native proteins, lipids, nucleic acids, and cofactors that form functional complexes.
Post Translational Modifications (PTMs)
Natural modifications that regulate kinase activity and interactions.
Signaling Networks
Dynamic feedback and crosstalk, including off-target enzymes that impact selectivity and potency.
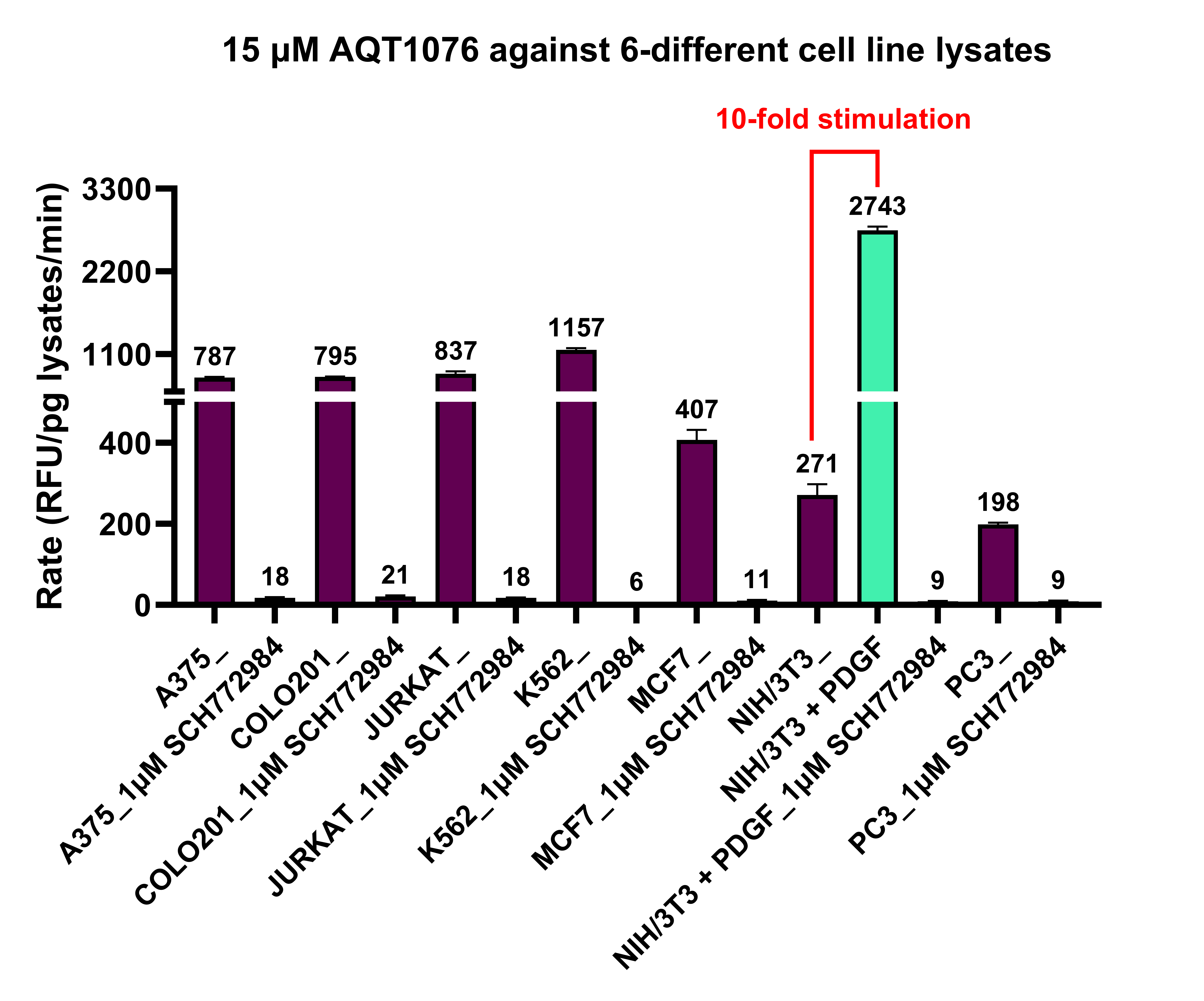
Our PhosphoSens™ Lysate Assays don’t just measure enzyme activity—they show how compounds behave in real cellular environments. View sample data that highlights pathway-specific kinase responses across multiple cell models, revealing insights you won’t see with traditional endpoint assays:
Stay Informed
Want to hear the latest about our technology? Be among the first to learn about our latest products and services.

x48(h)_v5_ES_Final.png)